Paralyzed man regains use of arms and hands after experimental stem cell therapy
Initial results offer hope for patients to reclaim independence after suffering severe spinal injury
September 12, 2016

Kris Boesen (credit: USC)
Doctors at the USC Neurorestoration Center and Keck Medicine of USC injected an experimental treatment* made from stem cells and other cells into the damaged cervical spine of a recently paralyzed 21-year-old man as part of a multi-center clinical trial.
Two weeks after surgery, Kristopher (Kris) Boesen began to show signs of improvement. Three months later, he’s able to feed himself, use his cell phone, write his name, operate a motorized wheelchair, and hug his friends and family. Improved sensation and movement in both arms and hands also make it easier for Kris to care for himself, and to envision a life lived more independently.
“Typically, spinal cord injury patients undergo surgery that stabilizes the spine but generally does very little to restore motor or sensory function,” explains Charles Liu, MD, PhD, director of the USC Neurorestoration Center. “With this study, we are testing a procedure that may improve neurological function, which could mean the difference between being permanently paralyzed and being able to use one’s arms and hands. Restoring that level of function could significantly improve the daily lives of patients with severe spinal injuries.”
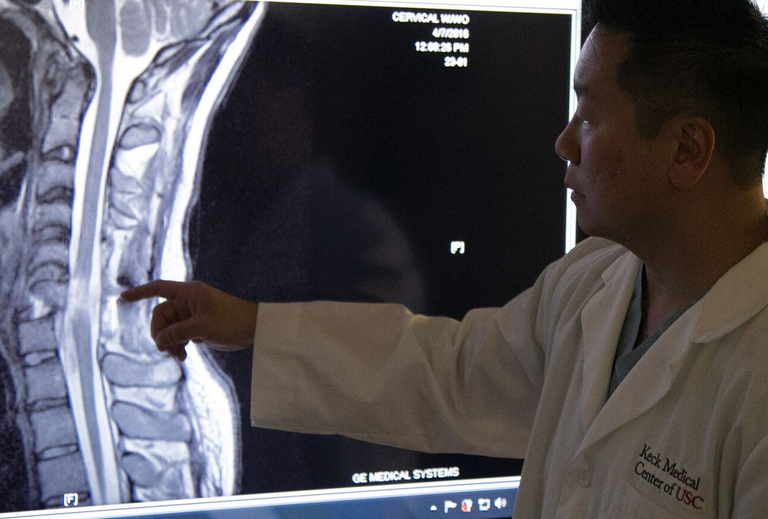
Dr. Liu points out cervical spine injury (credit: USC)
On March 6, Boesen suffered a traumatic injury to his cervical spine when his car fishtailed on a wet road, hit a tree and slammed into a telephone pole. Parents Rodney and Annette Boesen were warned there was a good chance their son would be permanently paralyzed from the neck down.
“As of 90 days post-treatment, Kris has gained significant improvement in his motor function, up to two spinal cord levels,” said Liu. “In Kris’ case, two spinal cord levels means the difference between using your hands to brush your teeth, operate a computer, or do other things you wouldn’t otherwise be able to do, so having this level of functional independence cannot be overstated.”
The pioneering surgery is the latest example of how the emerging fields of neurorestoration and regenerative medicine may have the potential to improve the lives of thousands of patients who have suffered a severe spinal cord injury.
Keck is one of six sites** in the U.S. authorized to enroll subjects and administer the clinical trial dosage. To qualify for the clinical trial, enrollees must be between the age of 18 and 69, and their condition must be stable enough to receive an injection of AST-OPC1 between the fourteenth and thirtieth days following injury.
* The stem cell procedure is part of a Phase 1/2a clinical trial that is evaluating the safety and efficacy of escalating doses of AST-OPC1 cells developed by Asterias Biotherapeutics. AST-OPC1 cells are made from embryonic stem cells by carefully converting them into oligodendrocyte progenitor cells (OPCs), which are cells found in the brain and spinal cord that support the healthy functioning of nerve cells. In previous laboratory studies, AST-OPC1 was shown to produce neurotrophic factors, stimulate vascularization and induce remyelination of denuded axons. All are critical factors in the survival, regrowth and conduction of nerve impulses through axons at the injury site in the “SCiStar” clinical trial according the researchers.
** The SCiStar clinical trial participating institutions include Indiana University in Indianapolis, Medical College of Wisconsin in Milwaukee, Rush University Medical Center in Chicago, Shepherd Center in Atlanta and, in California, Stanford University/Santa Clara Valley Medical Center, and Rancho Los Amigos/Keck Medicine of USC.
http://www.kurzweilai.net/paralyzed...nd-hands-after-experimental-stem-cell-therapy
Initial results offer hope for patients to reclaim independence after suffering severe spinal injury
September 12, 2016

Kris Boesen (credit: USC)
Doctors at the USC Neurorestoration Center and Keck Medicine of USC injected an experimental treatment* made from stem cells and other cells into the damaged cervical spine of a recently paralyzed 21-year-old man as part of a multi-center clinical trial.
Two weeks after surgery, Kristopher (Kris) Boesen began to show signs of improvement. Three months later, he’s able to feed himself, use his cell phone, write his name, operate a motorized wheelchair, and hug his friends and family. Improved sensation and movement in both arms and hands also make it easier for Kris to care for himself, and to envision a life lived more independently.
“Typically, spinal cord injury patients undergo surgery that stabilizes the spine but generally does very little to restore motor or sensory function,” explains Charles Liu, MD, PhD, director of the USC Neurorestoration Center. “With this study, we are testing a procedure that may improve neurological function, which could mean the difference between being permanently paralyzed and being able to use one’s arms and hands. Restoring that level of function could significantly improve the daily lives of patients with severe spinal injuries.”

Dr. Liu points out cervical spine injury (credit: USC)
On March 6, Boesen suffered a traumatic injury to his cervical spine when his car fishtailed on a wet road, hit a tree and slammed into a telephone pole. Parents Rodney and Annette Boesen were warned there was a good chance their son would be permanently paralyzed from the neck down.
“As of 90 days post-treatment, Kris has gained significant improvement in his motor function, up to two spinal cord levels,” said Liu. “In Kris’ case, two spinal cord levels means the difference between using your hands to brush your teeth, operate a computer, or do other things you wouldn’t otherwise be able to do, so having this level of functional independence cannot be overstated.”
The pioneering surgery is the latest example of how the emerging fields of neurorestoration and regenerative medicine may have the potential to improve the lives of thousands of patients who have suffered a severe spinal cord injury.
Keck is one of six sites** in the U.S. authorized to enroll subjects and administer the clinical trial dosage. To qualify for the clinical trial, enrollees must be between the age of 18 and 69, and their condition must be stable enough to receive an injection of AST-OPC1 between the fourteenth and thirtieth days following injury.
* The stem cell procedure is part of a Phase 1/2a clinical trial that is evaluating the safety and efficacy of escalating doses of AST-OPC1 cells developed by Asterias Biotherapeutics. AST-OPC1 cells are made from embryonic stem cells by carefully converting them into oligodendrocyte progenitor cells (OPCs), which are cells found in the brain and spinal cord that support the healthy functioning of nerve cells. In previous laboratory studies, AST-OPC1 was shown to produce neurotrophic factors, stimulate vascularization and induce remyelination of denuded axons. All are critical factors in the survival, regrowth and conduction of nerve impulses through axons at the injury site in the “SCiStar” clinical trial according the researchers.
** The SCiStar clinical trial participating institutions include Indiana University in Indianapolis, Medical College of Wisconsin in Milwaukee, Rush University Medical Center in Chicago, Shepherd Center in Atlanta and, in California, Stanford University/Santa Clara Valley Medical Center, and Rancho Los Amigos/Keck Medicine of USC.
http://www.kurzweilai.net/paralyzed...nd-hands-after-experimental-stem-cell-therapy
Last edited:
